Can’t-Miss Takeaways Of Info About How To Recover The Dissolved Solid In A Solution
Many alloys are solid solutions of one metal dissolved in another;
How to recover the dissolved solid in a solution. Answered by bobpursley 15 years ago 0 0 salt dissolved in water is a. Many alloys are solid solutions of one metal dissolved in another; Discuss the idea of water as the universal solvent.
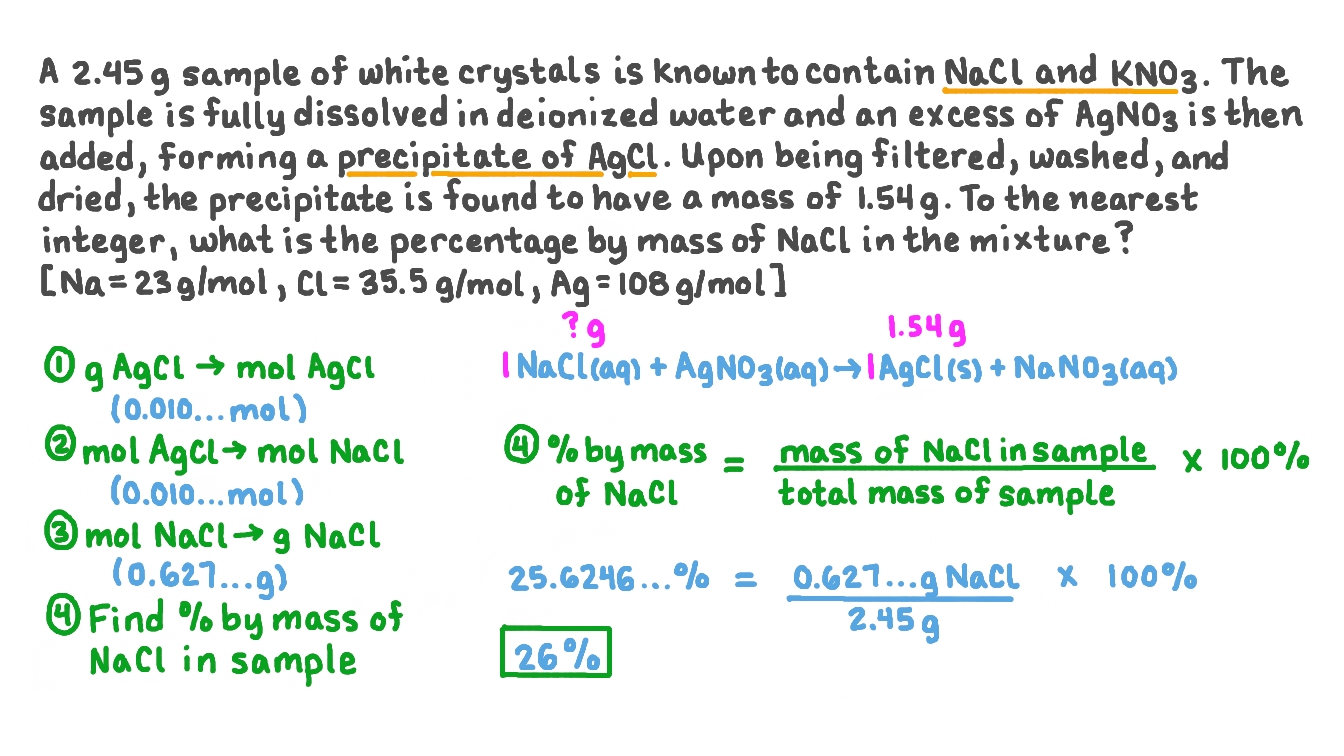
Some of the solid agcl continues to dissolve, but at the same. In this experiment, students use basic apparatus to evaporate and condense the water from copper (ii) sulfate solution. 3 answers asked by lyne 15 years ago 366 views 0 0 3 answers evaporation of trhe solvent?
The dissolved substances in an aqueous solution may be solids, gases, or other liquids. However, almost any gas, liquid, or solid can act as a solvent. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution.
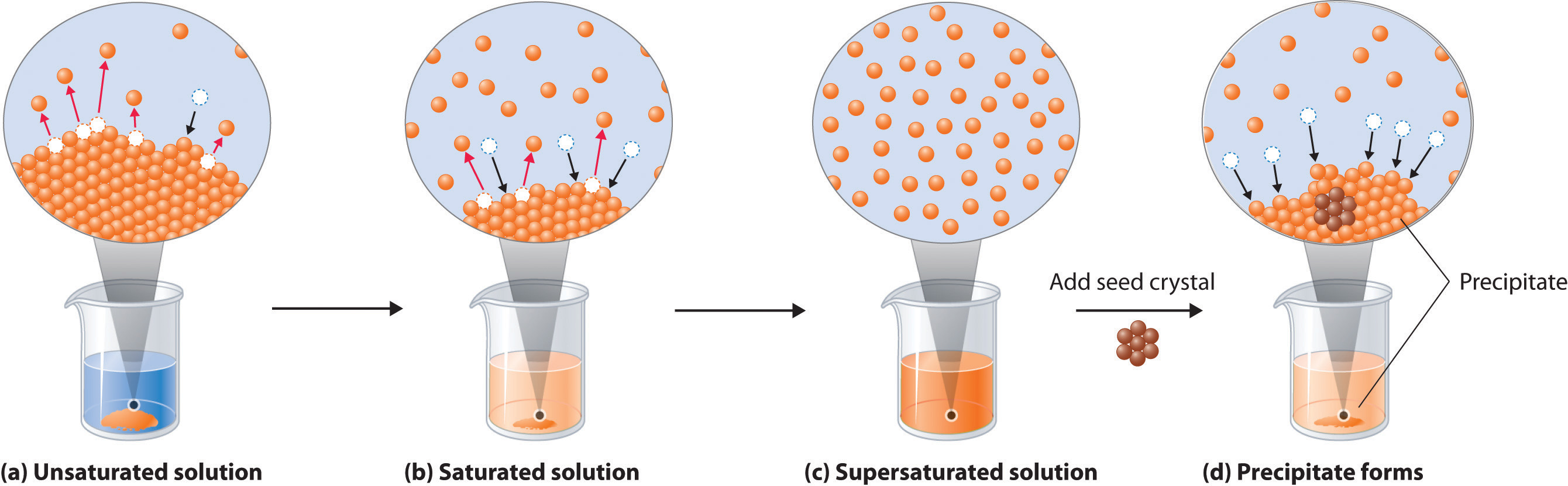
In order to be a true solution, a mixture must be stable. It includes all the key information about solutions and. The question is asking for sodium chloride, also known as table salt, to be recovered or separated from water after being dissolved in it, which means the technique the student.
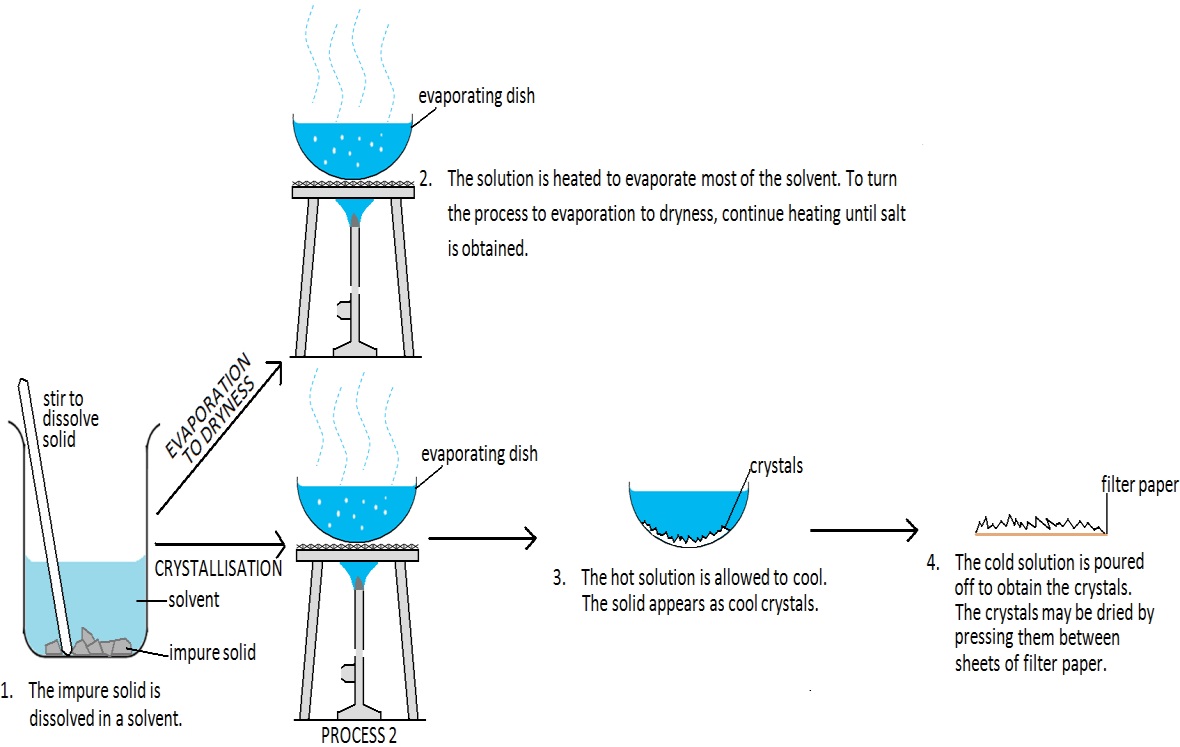
Explain why solutions form. Pour the mixture through the filter funnel. The method chosen depends upon the.
This is a simple introduction to aqueous solutions. Separating sand from salt water. Air is a gaseous solution, a.
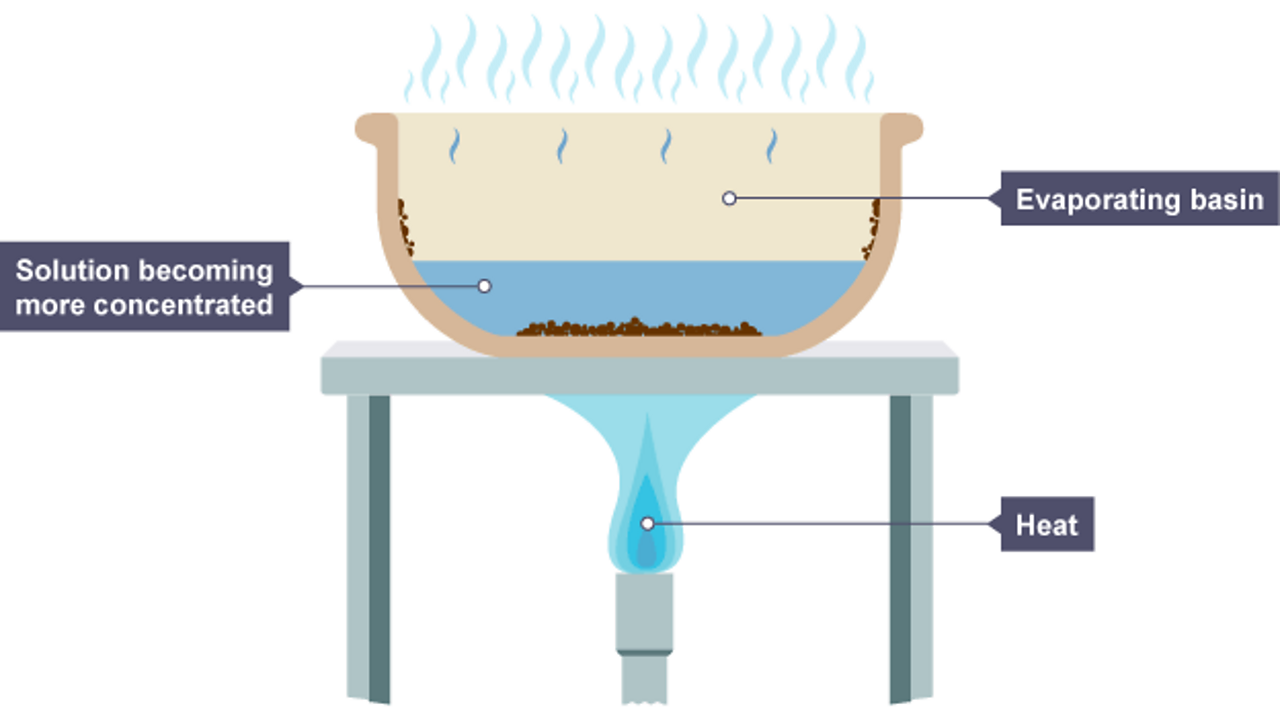
If the granules are not there, the liquid may ‘bump’, ie boil violently. Filter, wash with pure water, and dry recovering the dissolved solid from the solution we will just look at this in general terms, because it is pretty unlikely that you would want to. Each solution contains one component.
If it is not soluble, then the chemical will not dissolve and you can see it,. Let the water drain and leave the insoluble solid to dry. If a substance can dissolve into a solvent, it is soluble if it cannot dissolve, it is described as insoluble.
Evaporation can be used as a technique to separate the solid solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. In order to be a true solution, a mixture must be stable. Solutions and separations there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography.
Carry out equilibrium computations involving solubility, equilibrium expressions, and solute concentrations. When a small amount of solid potassium dichromate is added to water, the compound dissolves and dissociates to yield potassium ions and dichromate ions uniformly. Point out to the students the mode of action of the water.